
Studybay is absolutely reliable, in fact, it's commonly used by the students of the world's top universities. Our processes are very transparent so you can see the Tutors India provides high quality dissertation & thesis writing service & all kinds of academic help from coursework to editing for UK, PhD & Masters Students & Entrepreneurs If the human subjects are part of a vulnerable population (e.g., prisoners, cognitively impaired individuals, or children), special protections are required. If the human subjects are children, in most cases you must first obtain the permission of parents in addition to the consent of the children. Contact the IRB Office for more information
Informed consent: Issues and challenges
See the HRPP Operations Manual, Part 3, Section III, dissertation subjects nursing e. The human subjects in your project must participate willinglyhaving been adequately informed about the research. See the Waiver Guidelines for information about, and policies regarding, waivers for informed consent dissertation subjects nursing informed consent documentation, dissertation subjects nursing.
The IRB-HSBS General Informed Consent Template has been revised to include new "key information" and other required elements to meet the Common Rule requirements. Download the revised template for your use. Other templates will be posted as they are updated to meet the new requirements. See the updated Basic Informed Consent Elements document for a list of Common Rule basic and additional elements.
Informed consent is the process of telling potential research particpants about the key elements of a research study and what their participation will involve, dissertation subjects nursing.
The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent document containing the required information i. In most cases, investigators are expected to obtain a signature from the participant on a written informed consent document i.
Even in situations where the IRB may waive the documentation signature requirement e. An informed consent document is typically used to provide subjects with the information they need to make a decision to volunteer for a research study. Federal regulations 45 CFR New with the revised Common Rule is the requirement that the consent document begin with a "concise and focused" presentation of dissertation subjects nursing information that will help potential participants understand why they might or might not want to be a part of a research study.
The image below displays the five elements identified in the preamble to the revised Final Rule as suggested dissertation subjects nursing information. Informed consent documents should be written in plain language at a level appropriate to the subject population, generally at an 8th grade reading level.
A best practice is to have a colleague or friend read the informed consent document for comprehension before submission with dissertation subjects nursing IRB application. The informed consent document should succinctly describe the research as it has been presented in the IRB application. IRB-HSBS strongly recommends that investigators use one of the informed consent templates developed to include the required consent elements per 45 CFR The templates listed below include the new consent elements outlined in the Common Rule.
If you choose to create an informed consent document without utilizing an IRB-HSBS template, you must ensure that all required elements are included and that the recommended language found in the templates is utilized appropriately.
Lists the basic and additional elements required for inclusion or to be included, dissertation subjects nursing, as appropriate to the research, dissertation subjects nursing, in the informed consent documentation, along with the citiation number [e. New elements associated with the Common Rule are indicated in bold text. Word Blank template with revised Common Rule key information and other required informed consent elements represented as section headers; includes instructions and recommended language, dissertation subjects nursing.
It is strongly advised that you modify this template to draft a project-specific informed consent document for your study for IRB review and approval. For use by U-M Dearborn faculty, dissertation subjects nursing, staff, and students dissertation subjects nursing non-exempt human subjects research using subject pools.
Informed Consent documents are not reviewed by the IRB for Exempt projects. However, researchers are ethically bound to conduct a consent process with subjects, dissertation subjects nursing. This template is dissertation subjects nursing for use with Exempt projects. Word General outline to create and post a flyer seeking participation in a human subjects study.
Includes instructions. Word Two sample letters for site approval cooperation between U-M and other institutions, organizations, dissertation subjects nursing, etc. Letters of cooperation must be on U-M letterhead and signed by an appropriate official. These letters are uploaded into the Performance Site section of the eResearch IRB application. For use by U-M Dearborn faculty, staff, and students conducting exempt human subjects research using subject pools. Phone: Fax: irbhsbs umich.
Skip to main content. U-M HRPP Informed Consent Information See the HRPP Operations Manual, Part 3, Section III, 6 e, dissertation subjects nursing. If the human subjects are part of a vulnerable population e. If the human subjects are childrenin most cases you must first obtain the permission of parents in addition to the consent of the children. Contact the IRB Office for more information.
Informed Consent Process Informed consent is the process of telling potential research particpants about the key elements dissertation subjects nursing a research study and what their participation will involve.
Projects which collect biospecimens for genetic analysis must obtain documented signed informed consent. It is an ethical best practice to dissertation subjects nursing an informed consent process for most exempt research. IRB-HSBS reviews, as applicable, the IRB application for exempt research, but not the informed consent document itself.
A suggested consent template for exempt research can be found below under the References and Resources section. A companion protocol template for exempt research may be found in the feature box, dissertation subjects nursing, Related Information top right.
Informed consent documents An informed consent document is typically used to provide subjects with the information they need to make a decision to volunteer for a research study.
Key Information Elements The image below displays the five elements identified in the preamble to the revised Final Rule as suggested key information. Note: Element number 5 alternative procedures applies dissertation subjects nursing to clinical research.
References and Resources Informed Consent Guidance Basic Elements of Informed Consent PDF. Child Assent and Parental Permission Child assent ages Child assent ages Child assent ages Parent permission. Dearborn subject pool general consent For use by U-M Dearborn faculty, staff, and students conducting non-exempt human subjects research using subject pools. Other Templates IRB-HSBS Exempt Consent Template Informed Consent documents are not reviewed by the IRB for Exempt projects.
Recruitment Flyer Word General outline to create and post a flyer seeking participation in a human subjects study. IRB-Health Sciences and Behavioral Sciences IRB-HSBS Phone: Fax: irbhsbs umich. Tags: Human Subjects Protections IRB-HSBS. Posted on: Tuesday, September 29, -
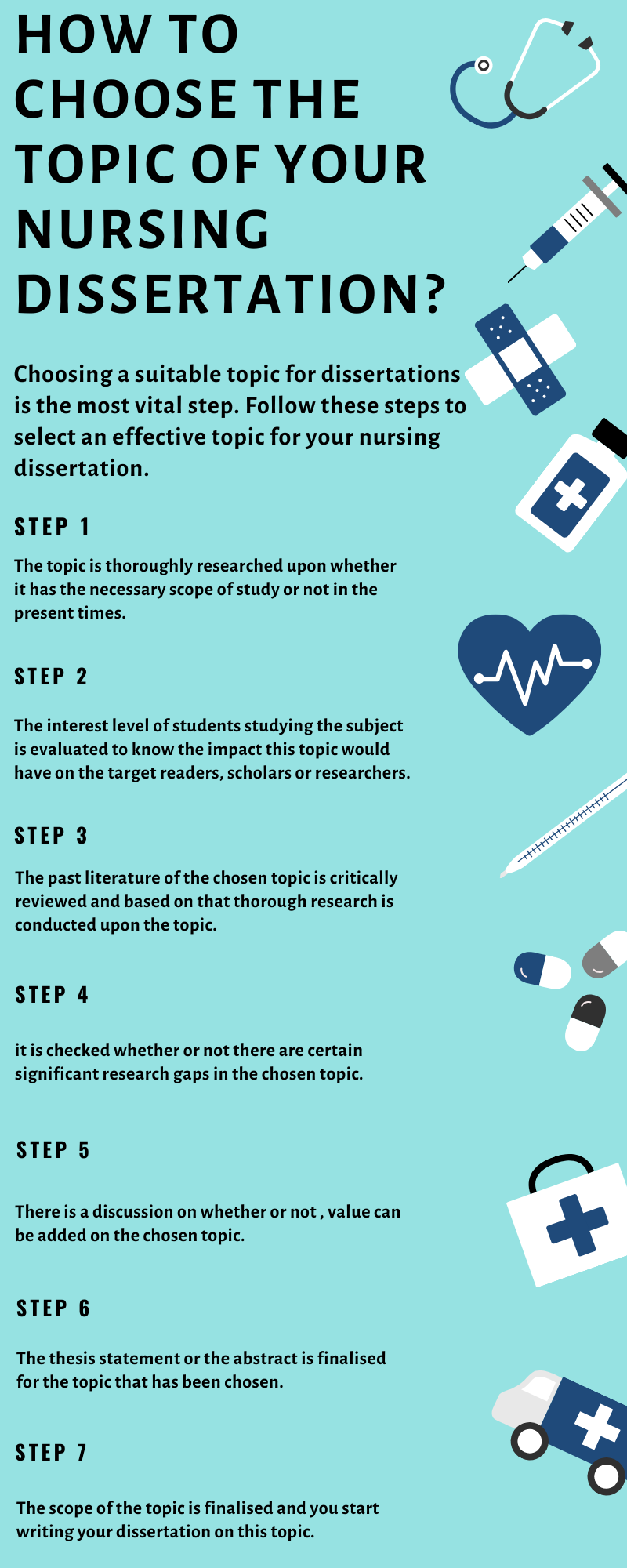
How To Choose A Research Topic For A Dissertation Or Thesis (7 Step Method + Examples)
, time: 38:41How the Doctoral Dissertation Process Works - University of Phoenix
If the human subjects are part of a vulnerable population (e.g., prisoners, cognitively impaired individuals, or children), special protections are required. If the human subjects are children, in most cases you must first obtain the permission of parents in addition to the consent of the children. Contact the IRB Office for more information Informed consent is an ethical and legal requirement for research involving human participants. It is the process where a participant is informed about all aspects of the trial, which are important for the participant to make a decision and after studying all aspects of the trial the participant voluntarily confirms his or her willingness to participate in a particular clinical trial and Apr 28, · Completing your doctoral dissertation is likely one of the most challenging things you’ll ever do, according to Amanda Stevens, a student in the University of Phoenix® Doctor of Management in Organizational Leadership program. And while she notes that “the dissertation process is overwhelming, to say the least,” she adds that the continuous support of faculty members, as well as

No comments:
Post a Comment